
- #Daftar online form how to#
- #Daftar online form manual#
- #Daftar online form registration#
- #Daftar online form license#
#Daftar online form registration#
Information on submission purpose of ”CAB Application/ Registration Fee” and Phone No. The bank draft shall be made payable to "KUMPULAN WANG PIHAK BERKUASA PERANTI PERUBATAN" and courier to The Authority will not accept cash payment. The payment shall be made through bank draft. The fee payable for the registration of a CAB: RM 8,000 The fee payable for an application of a CAB: RM 1,500 Please refer to Medical Device Regulation 2012 Fourth Schedule for the Requirements for the Registration of Conformity Assessment Bodies for further reference.Īpplication for the registration of CAB is fully online via our new upgraded online system 2.0.Īll applications for Conformity Assessment Body registration under Medical Device Act 2012 (Act 737) will be charged the application fee of RM 1,500 per submission and RM 8,000 upon approval for registration as registration fee.įees are to be charged to the applicant as stated in Table of Fees, Part IV, Fifth Schedule of Medical Device Regulation 2012. (iv) requirements on quality management system. (iii) requirements on independence and impartiality and (ii) requirements on resources and technical competency Detailed requirements for registration of CAB are as stipulated in Fourth Schedule of MDR2012 which includes: The requirements for registration of CAB are provided in Sections 10 and 11 Act 737 and Regulations 8, 9 and 10 of MDR2012. For further information on the requirement of QMS for medical device registration, please refer to Third Schedule of MDR2012. ISO 13485 certificate issued by registered CAB shall be submitted for registration of medical devices. Besides the list of CAB, you will also find other useful information. The list of CAB is available on our website. (d) Any additional information, particulars or documents required by the Authority under paragraph (3)(c) shall be provided by the applicant within thirty days from the date of request by the Authority (c) any additional information, particulars or documents as may be required by the Authority. (b) documents or information as specified in forms to be determined by the Authority and (a) application fee as specified in Fifth Schedule (3) An application for registration of a conformity assessment body shall be accompanied with the following: (2) An application for registration of a conformity assessment body shall be made in forms to be determined by the Authority (1) Any person who intends to be a conformity assessment body shall comply with the requirements as specified in Fourth Schedule and shall apply for registration to the Authority. Regulatory Information Regarding to Healthcare Institution.Notification for Export Only Medical Device.

Certificate Free Sale / Manufacturing Certificate.Clinical Research / Performance Evaluation.Demonstration for Marketing / Education.Post Market Survellence & Vigilance (PMSV).
#Daftar online form manual#
#Daftar online form how to#
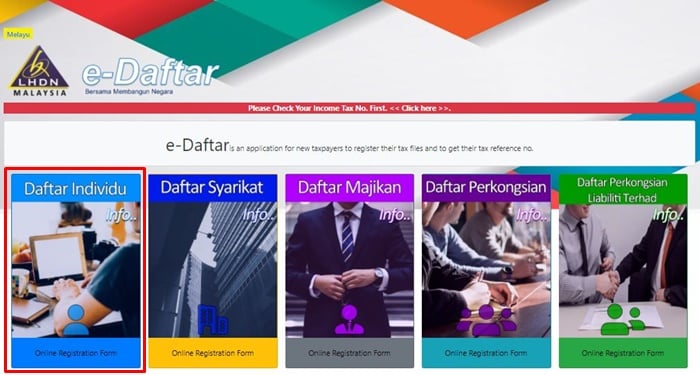
#Daftar online form license#


 0 kommentar(er)
0 kommentar(er)
